COGNITRUE BETA ANALYSIS
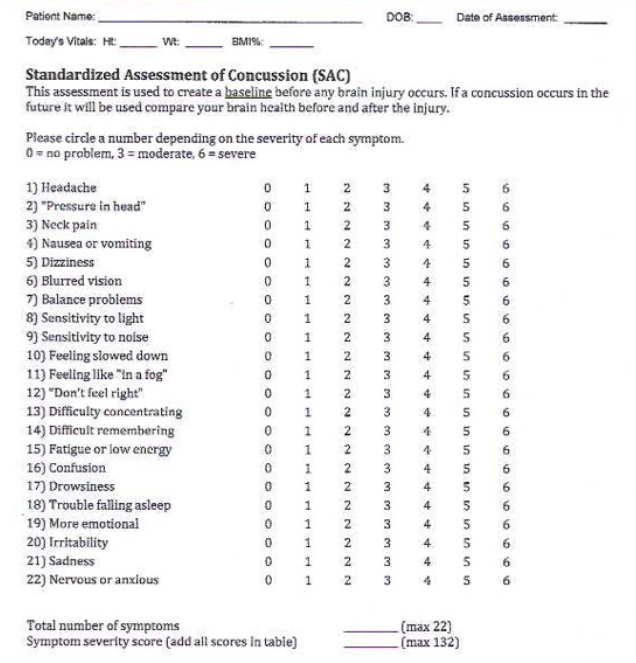
Thirty-one participants completed a beta test to measure the effectiveness of the CogniTrue® nootropic formulation on brain function. The beta test was conducted over three months from November 2024 through January 2025 and included participants from two midwestern states. The assessment tool was the Standardized Concussion Assessment form, ranking 21 items from 0 to 6. These questions related to pain and sensitivity, memory, mental focus, fatigue, and mental anxiety.
The participants were instructed to take the recommended dosage of 2 capsules twice per day for a period of three months and complete the assessment form each week.
The average improvement of the 31 participants was 61%.
The Standardized Concussion Assessment form is attached and consists of the following 22 items:
Headache, pressure in head, neck pain, nausea/vomit, dizziness, blurred vision, balance problems, sensitivity to light, sensitivity to noise, feeling slowed down, feeling “in a “fog,” “Don’t feel right,” difficulty concentrating, difficulty remembering, fatigue or low energy, confusion, drowsiness, trouble falling asleep, more emotional, irritability, sadness, and nervous or anxious.
Improvement was measured from the initial assessment to the final assessment. This provided an overall percentage improvement of total ranking of the severity of symptoms.
Three of the individuals were included in an extended study. These were either elderly or had multiple severe concussions with noted memory issues. The average improvement of this group was 32%.
Participants were asked to write in changes and improvements they experienced in their own words as they completed the standardized forms.
It was noted that some of the participants had lower scores in the first month. This may have been a form of detoxifying as the body was clearing out scaring. Most noticed improvement by the third month of using the product, many had significant results in the second month. Some had unusually stressful situations. While some of the potential participants discontinued due to this, others noted they felt better able to cope through the stressful periods. Others discontinued for reasons of lack of compliance, illness, too many other medications, and reaction to the supplement (mostly detox).
Examples of these comments are listed below:
“Memory recall is definitely improved”
“I am clearer and more relaxed.”
“I am not as anxious when I merge onto the freeway”
“It is easier for me to make managerial decisions at work”
“I was told I sound clearer than I ever have”
“I can remember names more quickly”
“People say I look younger and healthier”
“It takes less time for names and details to come to the surface. I can pull things up faster”
“I am not as scattered. This is working.”
“I can remember what I went into the other room to get!”
“I pulled up names from 55 years ago that I had completely lost”
“This is the best brain supplement I have ever used”
“I don’t search as long for words”
“I felt ‘clearer’ and had more emotional space to make requests that usually felt vulnerable”
“I get the feeling this supplement is making space for the piezoelectric charge in the matrix of my brain tissue”
“My sense of smell is better”
“I sleep better and have more capacity than when I started taking this supplement”
“I noticed a difference in two weeks. My retrieval system feels quicker and more fluid with less moments of forgetfulness.”
“Notable recall results in 3 weeks”
“Did not panic when I lost a credit card. Calmly found it.”
“More focused when driving.”
“Long term memory returning. Calmer, ADD symptoms much better”
“Able to cope with very stressful situations more effectively.”
Weekly Ranked Assessment
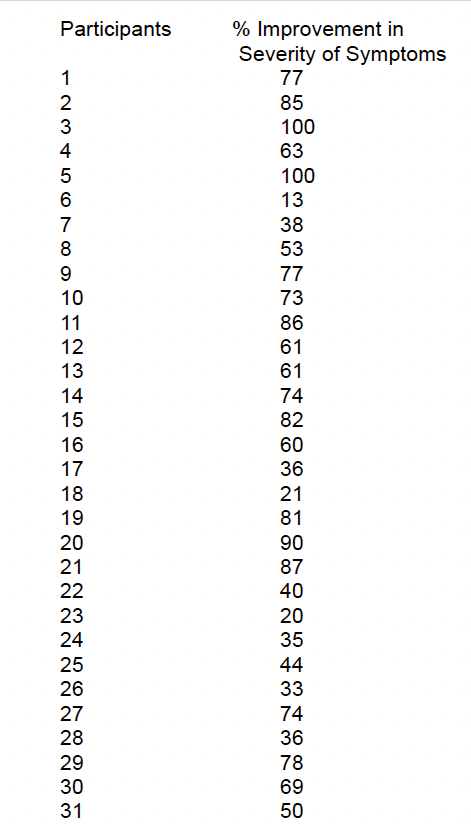
Average % Improvement for 31 Participants
61%
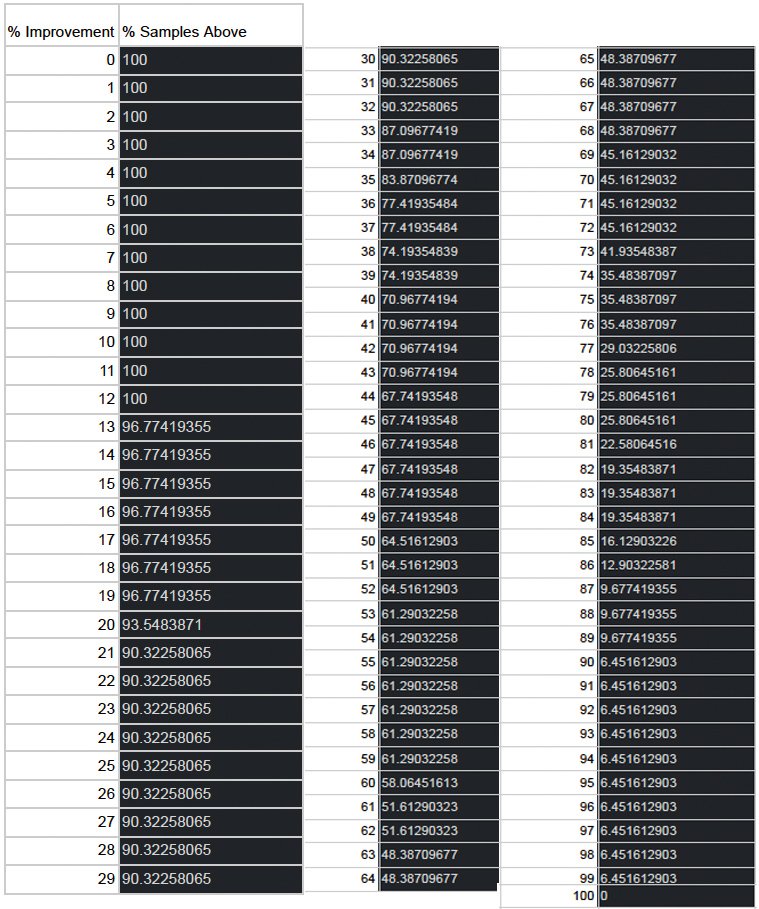
The following table shows the amount of % Improvement vs % of people that saw the improvement. E.g. 96.77 % people saw an improvement of 15%.